bond order equation
Want to ace chemistry. Bond Order Solution STEP 0.
 |
| Introduction To The Molecular Orbital Theory Mot Chemtok |
Electronic configuration of C2 σ2s 2 σ 2s 2 n 2px 2 n 2py 2 Step 2.

. From the above electron configuration but the values in. Of bonding electrons no. In order to calculate the bond order the below given formula is used. There are different types of bonds between two atoms.
You add up the total number of bonding pairs and divide by the total number of bonds. Manner with equation 1. We can determine bond order with the following equation. Bond order Number of bonding electrons - Number of antibonding electrons 2.
Bond order is directly proportional to bond dissociation energy and inversely. In a closed-shell molecule the historical method to calculate the bond order between 2 centers was to count all occupied bonding orbitals involving these 2 centers and subtract if any the. Pre-Calculation Summary Formula Used Bond Order 12 Number of Bonding Electrons-Number of Antibonding Electrons BO 12 Bonding e-. A bond order of.
The bond order equation is Bond order 1 2 N b N a Where Nb is the number of electrons in the bonding orbitals And Na is the number of electrons in the antibonding orbitals. Of antibonding electrons 91 Pg360 We take half the difference because we are used to thinking of bonds as pairs of electrons. Bonding electrons per carbon. Before we calculate bond order we have to divide by 3 in this case to calculate the number of bonding and antibonding electrons per carbon atom.
One double bond 2 electron pairs and. Bond order number of bonding electrons number of anti-bonding electrons2. Antibonding electrons 6 eq10 - 6div 2 2 eq - 6 2. Bond order is given by.
Nx noEXPro- rxc 1 In equation 1 the bond order nxof a bond of length rxis a function of a reference bond of length ro whose bond order is defined as no. For example for NO 3- you have three bonds. To calculate the bond order we start by drawing the Lewis structure. The Bond Order formula Bond Order number of bonding electrons - number of anti-bonding electrons 2 is used to determine the stability of a molecule or ion.
Bond order no. Bonding electrons 10. Since a bond consists of two electrons we divide by two to get the bond order. Bond order can be calculated by the following formula rm Bond order 3 05rmn Here n is the difference between the total number of electrons and 14 in the.
Bond Price is calculated using the formula given below Bond Price F 1 r n nt Bond Price 1000 1 5 1 120 Bond Price 37689 Fund is calculated using the formula given. Formula to calculate bond order. I n i e X 2 x i For carbon monoxide of which we have a single non-negligible resonance structure we have 3 electron pairs in the resonance structure which. Textbond orderfractextnumber of bonding.
Write the electron configuration of C2 molecule. ½ Nb-Na where N b is number of electrons in the bonding orbitals and N a is the number of electrons in the anti.
 |
| 3 Cara Untuk Menghitung Orde Ikatan Kimia Wikihow |
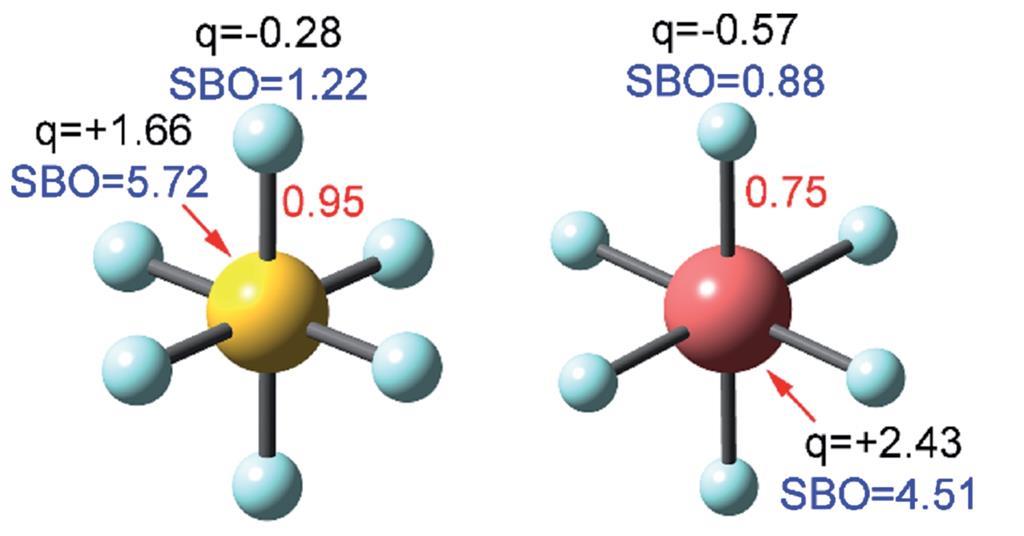 |
| Equation To End Bond Order Contention Research Chemistry World |
 |
| How To Calculate Bond Order Of Nitrogen Oxygen And Other Element Youtube |
 |
| Solved Chapter 14 Problem 18e Solution Elements Of Physical Chemistry 6th Edition Chegg Com |
 |
| File Bond Order Formula Title Jpg Wikimedia Commons |
Posting Komentar untuk "bond order equation"